Our team uses our experience and tools to help you optimize your product and processes,
full product life cycle from concept research to product introduction/deployment
to maintenance/support and continuous improvement.
We attack all phases with equal passion as illustrated by the studies below:
- BIG Business Innovation Award: Recognized for outstanding contributions to innovation in artificial intelligence and machine learning for healthcare and wellness platforms.
- SmartCEO Chief Technology Officer (CXO) Excellence Award: Honored for exceptional leadership and achievements in the role of Chief Technology and Operations Officer.
- SmartCEO Future 50 and Team Culture Awards: Acknowledged for contributions to organizational growth and fostering a positive team culture.
- PC Magazine Award Winning Imaging Technology Development and Product
- Patented Phase Change Ink Jet Technology Platform – Keynotes and $975M Acquisition
- Patented Bearing Free Variable Reluctance Linear Motors Drive MacroSonic Technology
- Best-of-Show Server Oil-less Sonic Cooling System
- Industry Recognition for Break-Through Sonic Power Mixing Processing Product Platform
- Award Winning Molecular Diagnostic Robotics Drives Mainstream Diagnostics Lab Adoption
- Electro-Magnetic Molecular Extraction Enables New Applications
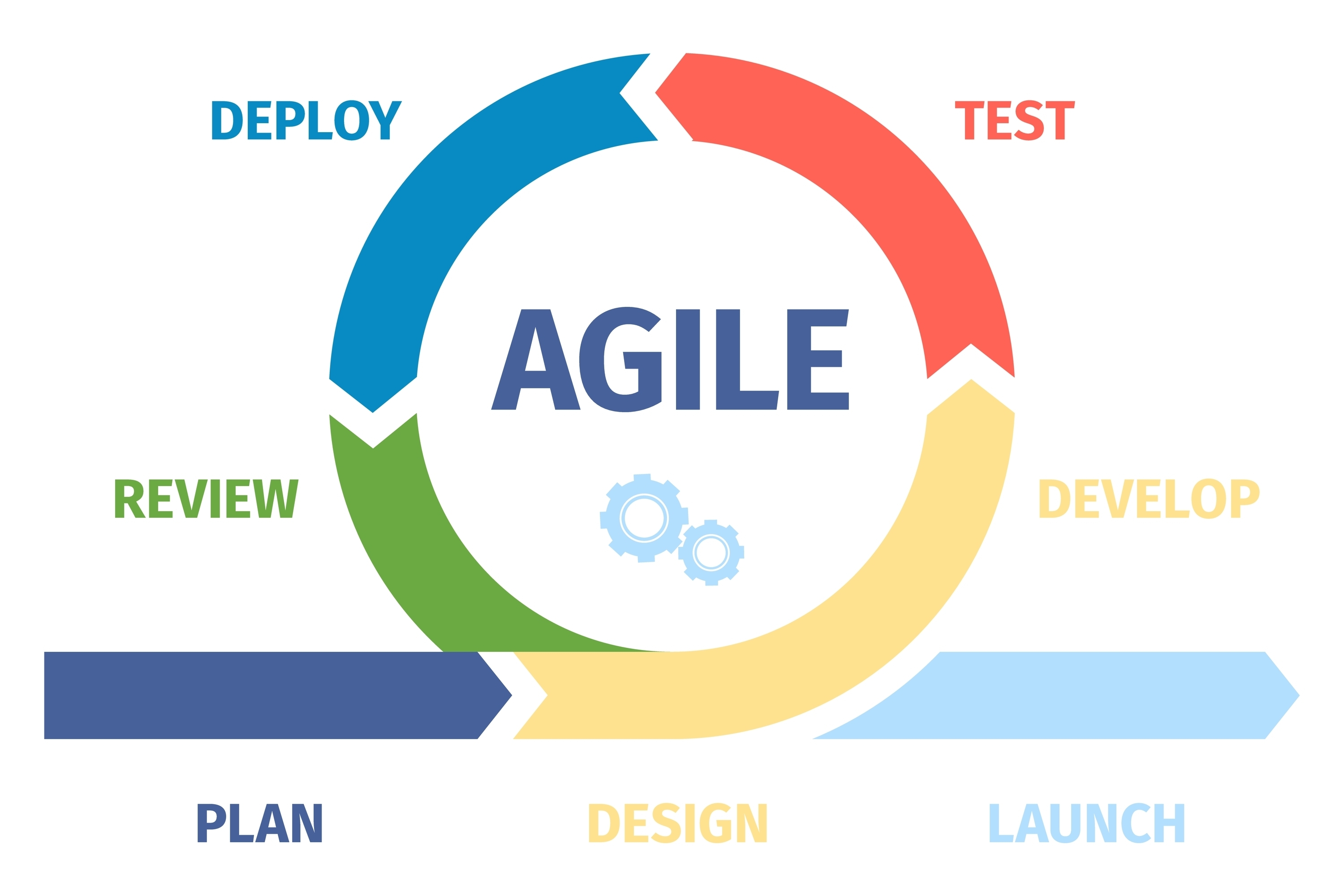
Agile Scrum Flow & Storyboard
Requirements
- Overall Requirements Management
- Business Requirements Analysis
- User Interviews & Focus Groups
- Questionnaires & Surveys
- Competitive Benchmarking Analysis
Concept
- Concept Development & Coordination
- Diagramming (UML) & Documentation
- Brainstorming Sessions & Background Research
- Concept Prototypes & Feasibility Testing
- Pugh Decision (Pugh) Matrices
Design
- Photoshop & Creative
- Design Drawings & Documentaion (SolidWorks)
- Data Structure & Flow Design & Schema (ERD)
- API Interfaces Specifications
- Detail Use Cases & Workflows (BDD/UML)
Development
- Behavior Driven Design/Development (BDD)
- Agile & Risk Driven Management
- Failure Modes & Effects Analysis (FMEA)
- Database Tracking
- Assembly Procedures
- Device Master Records
- Design History Files
- Embedded Control Code Implementation
- Production IT & ERD
- Lean/Kaizen & Six Sigma Techniques
Optimization
- Critical Parameter Analysis
- Tolerance and Stack-Up Analysis
- System Specification Development
- Module Specification Development
- Requirements Traceability Analysis
- Detail System Design
- Detail Mechanical Assembly
- Detail Component Design
- Embedded Control Architecture & Components
- Embedded Control Code Optimization
Verification, Validation
- Test Planning, Protocols, Execution & Reports
- Six Sigma Techniques
- Statistical & Analysis
- Design of Experiments (DOE)
- Instrumentation & Test Stand Development
- Test Execution & Automation
- User & Pre-Clinical (Medical Device) Validations
- Compliance & Safety Testing/Reporting
- Reliability Test and Growth Programs
- Machine Learning & Analytics
Continuous Improvement & Support
- Lean/Kaizen Techniques
- Production Drawings and Procedures
- Process Documentation & Validation
- User and Employee Training
- Structured Problem Solving & Six Sigma
- Continuous Improvement Programs
- Device Master Records & Design History Files
- Knowledge Base & Issue Tracking Tools
Regulatory & Quality Systems
- Quality Systems Development
- ISO 13485
- Audit Preparation (ISO/FDA)
- Design History/Technical File Preparation
- Submission Preparation & Writing
- Quality System Regulations
- Medical Device Directive
- Corrective & Preventive Actions (CAPAs)
Project Management & Tools
- Project Management Tools
- SDLC & Waterfall
- Agile (Kanban/Scrum, Jira)
- Requirements Tracking & Traceability
- Quality System Tracking
- Training Simulations
- Verification & Validation Packages
- Compliance/Clearance Submission & Audit Support