Let us help you optimize your embedded software and controller products and product life cycle
from concept research to product introduction/deployment
to maintenance/support and continuous improvement.
- PC Magazine Award Winning Imaging Technology Development and Product
- Patented Phase Change Ink Jet Technology Platform – Keynotes and $975M Acquisition
- Patented Bearing Free Variable Reluctance Linear Motors Drive MacroSonic Technology
- Best-of-Show Server Oil-less Sonic Cooling System
- Industry Recognition for Break-Through Sonic Power Mixing Processing Product Platform
- Award Winning Molecular Diagnostic Robotics Drives Mainstream Diagnostics Lab Adoption
- Electro-Magnetic Molecular Extraction Enables New Applications
Endoscopic Cryosurgery Medical Device

A cryosurgery system for application of medical-grade liquid nitrogen via a small,
low pressure, open tipped catheter.
The system includes a console, touch panel computer, cryogen module,
suction module, electronics module, and disposable spray kit.
Features include cryogen flow cryogen flow control and consistency,
integrated suction pump with self-checks,
pressure sensing during a treatment,
and novel catheter designs.
Macrosonix Powder Mixing

Sonic power mixing processing product platform.
Industry recognition for break-through sonic powder mixing processing product platform.
Applied fluidic simulations to characterize the activity and drove optimized design of experiments (DOE) to improve product performance and reliability.
Molecular Diagnostic Robotic Automation
 BD Howe Award
BD Howe Award
Award winning molecular diagnostic robotics drives mainstream diagnostics lab adoption.
Robotic instrument for molecular diagnostic testing.
Implemented concept characterization and design of experiment tools to obtain rapid FDA clearance and to
secure multi-million dollar contract with leading national reference laboratory.
Developed customized requirements management tool for improved traceability.
Electro-Magnetic Molecular Extraction
 BD Howe Award
BD Howe Award
Electro-magnetic molecular extraction enables new applications.
Enhanced the automated sample processing and furthered the contract with national reference laboratory including over 100 placements in testing laboratories across the US.
Applied reliability growth tools to increase the system Mean Time Between Failure (MTBF).
Tektronix Phaser Platform
 Howard Vollum Award; Presidents Award
Howard Vollum Award; Presidents Award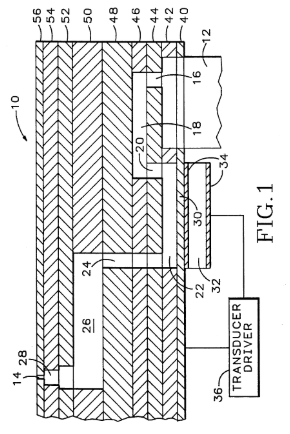
Patented Phase Change Ink Jet Technology Platform yields keynotes and $975M acquisition
Patented phase change ink jet technology platform.
Continuous improvement of existing key technology.
Used computer simulations and design of experiments to achieve variable resolution and high speed stability and consistency.
Imaging & Printing Technology
 PC Magazine Award Winning
PC Magazine Award Winning
Patented phase change ink jet technology platform.
Developed breakthrough product for high speed office printing by using acoustic simulations to drive an optimized product.
Six sigma tools applied to produce robust and reliable printer with patented technology.
Linear Resonance Compressors

Linear resonance compressors drive Macrosonic technology.
Re-characterized the method for for compressing to achieve best in class products.
Macrosonic Cooling System
 Best-of-Session
Best-of-Session
Generated concept for variable capacity vapor compression cooling and further optimized with design of experiments.
Linear Motor Patented Design

Patented bearing free variable reluctance linear motors drives.